01dragonslayer
Iron Killer
Mad Referrer
Jacked Immortal
EG Auction Sniper
VIP Member
Shout Master
Mutated
Fully Loaded
- EG Cash
- 1,113,693
Biohacking has been a huge trend the past few years, including everything from how to improve cognitive function and sleep quality to more simple things such as what to eat for better-looking hair.
One of the most intriguing areas of biohacking revolves around increasing lifespan, with some believing that with enough self-hacking they can live beyond 130 years... Naturally.
The keys to a longer lifespan that you probably already know about and may (or may not) do include:
You read that right the ultimate biohack to a happier, healthier life might be through self-destruction. If you have no idea what we’re talking about, or think we’ve just gone plan batshit crazy, then stick around to learn about one of the hottest topics in the research community right now in autophagy.
Autophagy is now known to play a role in many different diseases including cancer, neurodegeneration, aging, heart disease, infection, and autoimmune diseases such as Crohn’s disease or rheumatoid arthritis. [7]
Autophagy helps maintains homeostasis by sequestering portions of the cell that are considered old, not functioning properly, or dying and then breaking them down and reusing those pieces to build new structures for the cell. It also plays a vital role in preserving health, as a number of degenerative diseases are linked to an accumulation of damaged cells, including dementia.
In other words, autophagy is kind of like the ultimate detox -- your body cleanses the toxins and rebuilds itself anew. and self-regeneration all wrapped in one tidy package.
Autophagy isn’t exclusive to humans either. In fact, there are 32 different autophagy-related genes that have been identified, mainly through genetic screening in yeast and rats. There’s even been evidence of these autophagy genes in slime mold, plants, worms, flies and other mammals, which highlights the importance of the process of autophagy may have in sustaining life.
Now, let’s take a little bit deeper look at how autophagy really works inside the cell.
P62 is activated when metabolic byproducts that cause cell damage known as reactive oxygen species (ROS) are detected. P62 collects these damaged goods in order to help your body handle stress and thus kickstarts the process of autophagy. [4]
Autophagy occurs in the cytoplasm (the thick solution that fills the inside of all cells) and begins with a double-membraned structure called the isolation membrane or phagophore, though the exact process of how the phagophore forms is still yet to be understood. [1] Researchers suspect the lipid bilayer is formed by contributions from the endoplasmic reticulum and/or the trans-Golgi, but the matter is still up for debate. [2][3]
Origin story of the phagophore aside...
The phagophore gradually expands by acquiring lipids and then seals to become a double-membraned vesicle called an autophagosome.
The autophagosome then “collects the garbage” that’s going to be processed, degraded, and finally recycled. As for what the “cargo” that the autophagosome-trash collector gathers, the list includes any and all of the following:
 Membrane structures and organelles in the autophagic pathway. [4]
Membrane structures and organelles in the autophagic pathway. [4]
Membrane transport proteins in the lysosome called permeases then export the individual amino acids and other decomposed bits resulting from the breakdown back out into the cytoplasm. These recycled parts are then reused to build new macromolecules and for metabolism.
In other words, autophagy is essentially a cellular recycling facility that gets rid of the “bad” stuff, makes more of the “good” stuff and improves energy efficiency via ATP generation. This makes autophagy act as a sort of autoregulator for damage done by non-functional organelles and proteins that helps preserve life and serve as a means to avoid cell self-destruction, a.k.a apoptosis.
Sounds pretty scary, doesn’t it?
In certain scenarios it can be, but make no mistake, apoptosis is an essential component of an organism’s normal growth and development. So, how is does it relate to autophagy?
Well, for starters there’s a pretty big difference between the two. In apoptosis, the whole kitten kaboodle goes down, meaning for all intents and purposes self-detonates. In autophagy, non-functioning components of the cell are broken down and recycled to produce newer, better functioning parts so as to ensure the survival of that cell.
So in this context, it would appear that apoptosis and autophagy are at odds. One results at the end of a cell, while the other exists to prolong the life of the cell and combats the effects of aging and stress.
Furthermore, scientists believe that autophagy is somewhat ”selective” when it comes to which organelles, protein aggregates, and ribosomes are recycled. While apoptosis is the outright destruction of the entire cell, carried out for any number of reasons.
Cases of “beneficial” apoptosis occur when certain structures are no longer needed as part of an organism's natural growth and development. Case in point, mouse paws, which are formed as a direct result of cell death that occurs during embroyonic development. [6]
Mouse paws begin as spade-like structures and when certain portion die off, gaps are created between the “fingers” of paws leading to the shape we know so well today.
Moreover, apoptosis also helps regulate the total number of cells in the body. For example, cell death regulates the number of nerve cells to match the number of target cells requiring innervation in the central nervous system. [6] Another common example of apoptosis occurring in health functioning people is the programmed cell death of billions of cells in the bone marrow and intestines every hour. [6]
So, when taking these factors into account, the idea of cells essentially “offing” themselves doesn’t seem like a bad thing, entirely. You do enjoy having fingers and toes after all, don’t you?
Diving deeper into the relationship between autophagy and apoptosis, it appears that they’re not so diametrically opposed as some recent research indicates that autophagy may actually be a catalyst for apoptosis. [7]
Here’s a graph showing the inner workouts for those of you interested in the cellular biology:
 Connections between the autophagic and apoptotic processes. [7]
Connections between the autophagic and apoptotic processes. [7]
When looking even further into the connections between the two processes, researchers have identified several proteins that serve key roles in each:
 Table 1: Proteins with dual roles in autophagy and apoptosis. [7]
Table 1: Proteins with dual roles in autophagy and apoptosis. [7]
Several studies have noted that in certain situations, autophagy can encourage cell death, but the reason is not quite clear. [8][9][10] It could be that autophagy may kill a cell by destroying specific component required for cell survival (i.e. mitochondria) or by “inadvertently” killing the cell by degrading so many components to the point that the cell can no longer survive.
While autophagy may not be directly responsible for killing the cell, it certainly appears that it can indirectly cause apoptosis. This has led researchers to ponder does autophagy prevent death or cause it, as it’s been shown to play a role in the treatment of cancer and the progression of Alzheimer’s disease (where there is a severe lack of autophagy). [11]
Suffice it to say that the relationship between apoptosis and autophagy is complex and we have yet to gain a firm grasp on it, but it may be that autophagy can sustain life through apoptosis-mediated cell death in that autophagy protects cells that we want to keep, while destroying the inner components of cells (and indirectly activating apoptosis) we want to kill or remove (i.e. cancer cells). In other situations, cells can switch between apoptosis and autophagy in a mutually exclusive manner. [12]
See what we mean by saying the relationship is “complex”?
If that’s not enough to make your head spin, there’s not one singular type of autophagy either, but three different kinds.
In macro-autophagy, the “cargo” we discussed earlier is delivered to the lysosome through the use of the double membrane-bound vesicle called the autophagosome. The autophagosome then fuses with the lysosome to form an autolysosome. This is the kind of autophagy we’ve been discussing in this article for the most part.
In micro-autophagy, the “cargo” is directly absorbed by the lysosome through “invagination” of the lysosomal membrane. Essentially, the lysosome folds itself inside out to engulf the contents to be recycled.
Now, both macro-autophagy and micro-autophagy can engulf the large structures in the cell through selective as well as non-selective means. However, in chaperone-mediated autophagy, targeted proteins set for recycling are transported across the lysosomal membrane with the aid of a “chaperone” protein, such as Hsc-70. These “chaperone” proteins are recognized by the lysosomal membrane protein 2A (LAMP-2A), which leads to their unfolding and breakdown.
Now, for the purposes of this article, it’s not really necessary to understand the three types of autophagy. This just serves to explain all the different means your cells use to eat themselves. For this article, anytime you see “autophagy”, we’re referring to the macro kind.
Basically, autophagy is a response to stress.
How that stress is imposed on the body can be one of any number of external factors, including:
As you know, muscle growth and repair is mediated by the mechanistic target of rapamycin (mTOR) pathway in the body. When you eat protein, this pathway is activated and without getting into the incredibly complex physiology of how it happens (it would take WAY too long), autophagy is essentially deactivated.
Even small amounts of amino acids (~10 grams) or glucose can inhibit autophagy, as both glucose and certain amino acids can raise insulin, which is a principle negative regulators of autophagy. [13] What this means, is that even a small meal or sipping on your beloved BCAA can blunt the autophagic process.
While it may sound strange, if you want to maximize autophagy, you’re going to have to put your gains on hold for a bit, as autophagy is induced by mTOR inactivated, which is caused by nutrient deprivation, specifically protein.[29]
Fasting also causes a significant drop in certain growth factors, such as insulin-like growth factor 1 (IGF-1), which also serves as a signal to the body to activate autophagy.
How long do you need to fast?
Research in mice shows that at least 24-48 hours is needed to significantly upregulate autophagy. Other research notes that a 12 hour fast was able to upregulate hepatic autophagy in mice, but other tissues were not studied.
Now, we’re not suggesting you experiment with self-suffocation, but depriving your body of oxygen is a powerful stressor that can trigger the cell recycling process.
We can’t think of a more enjoyable form of stressing the body than intense exercise. High-intensity training such as heavy weight lifting or interval training (HIIT) subject the body to incredible stress, and in response to this stress, autophagy kicks in to repair and fortify your cells so that they are stronger and more durable the next time they encounter this type of stress.
One particular study engineered the test subjects (mice) to have glowing green autophagosomes (yes this really happens) to test the effects of intense exercise on autophagy. Researchers noted that the rate of self-eating was significantly increased after 30 minutes of running, and it continued to increase up to 80 minutes. [16]
Now, this study was done in mice, and we don’t know how long or intense the training must be in humans, yet, but clearly, there is a link between exercise and autophagy. Some human research has noted that short 20-minute sub-maximal aerobic exercise (81% VO2 max) did not exert significant effects on autophagy in skeletal muscle, but ultra-endurance exercise did. [22][23][24] So, until more research is done, the link between exercise intensity and duration and autophagy is still unclear.
How does keto relate to autophagy?
Remember, insulin and activation of mTOR are two of the biggest autophagy inactivators, so by going keto or extremely low carb with your diet, you keep insulin levels very low and promote autophagy.
This might also be a way to increase autophagy without having to full on fast.
While this hasn’t been extensively studied in humans, restricting your protein intake substantially (i.e. only consuming 25-30 grams PER day) for a week may activate autophagy by causing a drop in insulin-like growth factor-1 (IGF-1) which reduces the transfer of glucose into the cell, resulting in autophagy activation.
Autophagy is the ultimate stress-response mechanism in the body, one that can be activated a number of ways, but all involve stress in some form or another. While none of us enjoy stressing our bodies unnecessarily, it’s pretty clear that to live longer and be stronger, stress is required.
Starvation and exercise are the two strongest stressors in the body, use one or use both, just make sure you provide adequate stress to reap the numerous benefits of autophagy and eat yourself to a stronger you!
One of the most intriguing areas of biohacking revolves around increasing lifespan, with some believing that with enough self-hacking they can live beyond 130 years... Naturally.
The keys to a longer lifespan that you probably already know about and may (or may not) do include:
- Eating a healthy diet
- Getting adequate sleep
- Limiting stress
- Exercising regularly
- Maintaining a healthy body composition
You read that right the ultimate biohack to a happier, healthier life might be through self-destruction. If you have no idea what we’re talking about, or think we’ve just gone plan batshit crazy, then stick around to learn about one of the hottest topics in the research community right now in autophagy.
What is Autophagy?
Autophagy (aw-tah-fuh-gee) is a normal physiological process in the body that involves the destruction of cells. Derived from the Greek words auto (“self”) and phagy (“eating”), autophagy is literally translated as “eating of self”, and it’s essentially your body’s natural way of cleaning out damaged cells and toxins, which helps you then regenerate new, healthy cells.Autophagy is now known to play a role in many different diseases including cancer, neurodegeneration, aging, heart disease, infection, and autoimmune diseases such as Crohn’s disease or rheumatoid arthritis. [7]
Autophagy helps maintains homeostasis by sequestering portions of the cell that are considered old, not functioning properly, or dying and then breaking them down and reusing those pieces to build new structures for the cell. It also plays a vital role in preserving health, as a number of degenerative diseases are linked to an accumulation of damaged cells, including dementia.
In other words, autophagy is kind of like the ultimate detox -- your body cleanses the toxins and rebuilds itself anew. and self-regeneration all wrapped in one tidy package.
Autophagy isn’t exclusive to humans either. In fact, there are 32 different autophagy-related genes that have been identified, mainly through genetic screening in yeast and rats. There’s even been evidence of these autophagy genes in slime mold, plants, worms, flies and other mammals, which highlights the importance of the process of autophagy may have in sustaining life.
Now, let’s take a little bit deeper look at how autophagy really works inside the cell.
How Does Autophagy Work?
Autophagy is an evolutionary mechanism that’s enabled us to survive the various hazards of life. It’s initiated by small adaptations in a protein called p62.P62 is activated when metabolic byproducts that cause cell damage known as reactive oxygen species (ROS) are detected. P62 collects these damaged goods in order to help your body handle stress and thus kickstarts the process of autophagy. [4]
Autophagy occurs in the cytoplasm (the thick solution that fills the inside of all cells) and begins with a double-membraned structure called the isolation membrane or phagophore, though the exact process of how the phagophore forms is still yet to be understood. [1] Researchers suspect the lipid bilayer is formed by contributions from the endoplasmic reticulum and/or the trans-Golgi, but the matter is still up for debate. [2][3]
Origin story of the phagophore aside...
The phagophore gradually expands by acquiring lipids and then seals to become a double-membraned vesicle called an autophagosome.
The autophagosome then “collects the garbage” that’s going to be processed, degraded, and finally recycled. As for what the “cargo” that the autophagosome-trash collector gathers, the list includes any and all of the following:
- Damaged organelles
- Protein aggregates
- Ribosomes
- Bacteria
- Viruses
 Membrane structures and organelles in the autophagic pathway. [4]
Membrane structures and organelles in the autophagic pathway. [4]Membrane transport proteins in the lysosome called permeases then export the individual amino acids and other decomposed bits resulting from the breakdown back out into the cytoplasm. These recycled parts are then reused to build new macromolecules and for metabolism.
In other words, autophagy is essentially a cellular recycling facility that gets rid of the “bad” stuff, makes more of the “good” stuff and improves energy efficiency via ATP generation. This makes autophagy act as a sort of autoregulator for damage done by non-functional organelles and proteins that helps preserve life and serve as a means to avoid cell self-destruction, a.k.a apoptosis.
Autophagy and Apoptosis -- An Interesting Relationship
Apoptosis -- programmed cell death.Sounds pretty scary, doesn’t it?
In certain scenarios it can be, but make no mistake, apoptosis is an essential component of an organism’s normal growth and development. So, how is does it relate to autophagy?
Well, for starters there’s a pretty big difference between the two. In apoptosis, the whole kitten kaboodle goes down, meaning for all intents and purposes self-detonates. In autophagy, non-functioning components of the cell are broken down and recycled to produce newer, better functioning parts so as to ensure the survival of that cell.
So in this context, it would appear that apoptosis and autophagy are at odds. One results at the end of a cell, while the other exists to prolong the life of the cell and combats the effects of aging and stress.
Furthermore, scientists believe that autophagy is somewhat ”selective” when it comes to which organelles, protein aggregates, and ribosomes are recycled. While apoptosis is the outright destruction of the entire cell, carried out for any number of reasons.
Cases of “beneficial” apoptosis occur when certain structures are no longer needed as part of an organism's natural growth and development. Case in point, mouse paws, which are formed as a direct result of cell death that occurs during embroyonic development. [6]
Mouse paws begin as spade-like structures and when certain portion die off, gaps are created between the “fingers” of paws leading to the shape we know so well today.
Moreover, apoptosis also helps regulate the total number of cells in the body. For example, cell death regulates the number of nerve cells to match the number of target cells requiring innervation in the central nervous system. [6] Another common example of apoptosis occurring in health functioning people is the programmed cell death of billions of cells in the bone marrow and intestines every hour. [6]
So, when taking these factors into account, the idea of cells essentially “offing” themselves doesn’t seem like a bad thing, entirely. You do enjoy having fingers and toes after all, don’t you?
Diving deeper into the relationship between autophagy and apoptosis, it appears that they’re not so diametrically opposed as some recent research indicates that autophagy may actually be a catalyst for apoptosis. [7]
Here’s a graph showing the inner workouts for those of you interested in the cellular biology:
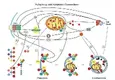 Connections between the autophagic and apoptotic processes. [7]
Connections between the autophagic and apoptotic processes. [7]When looking even further into the connections between the two processes, researchers have identified several proteins that serve key roles in each:
 Table 1: Proteins with dual roles in autophagy and apoptosis. [7]
Table 1: Proteins with dual roles in autophagy and apoptosis. [7]Several studies have noted that in certain situations, autophagy can encourage cell death, but the reason is not quite clear. [8][9][10] It could be that autophagy may kill a cell by destroying specific component required for cell survival (i.e. mitochondria) or by “inadvertently” killing the cell by degrading so many components to the point that the cell can no longer survive.
While autophagy may not be directly responsible for killing the cell, it certainly appears that it can indirectly cause apoptosis. This has led researchers to ponder does autophagy prevent death or cause it, as it’s been shown to play a role in the treatment of cancer and the progression of Alzheimer’s disease (where there is a severe lack of autophagy). [11]
Suffice it to say that the relationship between apoptosis and autophagy is complex and we have yet to gain a firm grasp on it, but it may be that autophagy can sustain life through apoptosis-mediated cell death in that autophagy protects cells that we want to keep, while destroying the inner components of cells (and indirectly activating apoptosis) we want to kill or remove (i.e. cancer cells). In other situations, cells can switch between apoptosis and autophagy in a mutually exclusive manner. [12]
See what we mean by saying the relationship is “complex”?
If that’s not enough to make your head spin, there’s not one singular type of autophagy either, but three different kinds.
Three Ways to Eat Yourself (a.k.a. 3 Types of Autophagy)
The three types of autophagy are:- Macro-autophagy
- Micro-autophagy
- Chaperone-mediated autophagy
In macro-autophagy, the “cargo” we discussed earlier is delivered to the lysosome through the use of the double membrane-bound vesicle called the autophagosome. The autophagosome then fuses with the lysosome to form an autolysosome. This is the kind of autophagy we’ve been discussing in this article for the most part.
In micro-autophagy, the “cargo” is directly absorbed by the lysosome through “invagination” of the lysosomal membrane. Essentially, the lysosome folds itself inside out to engulf the contents to be recycled.
Now, both macro-autophagy and micro-autophagy can engulf the large structures in the cell through selective as well as non-selective means. However, in chaperone-mediated autophagy, targeted proteins set for recycling are transported across the lysosomal membrane with the aid of a “chaperone” protein, such as Hsc-70. These “chaperone” proteins are recognized by the lysosomal membrane protein 2A (LAMP-2A), which leads to their unfolding and breakdown.
Now, for the purposes of this article, it’s not really necessary to understand the three types of autophagy. This just serves to explain all the different means your cells use to eat themselves. For this article, anytime you see “autophagy”, we’re referring to the macro kind.
What Causes Autophagy?
The simplest way to describe autophagy might be an adaptation to changing conditions. In other words, it is a way for your body to assess, recovery, and adapt to stress fortifying you from the inside so you’re stronger on the outside.Basically, autophagy is a response to stress.
How that stress is imposed on the body can be one of any number of external factors, including:
Nutrient Deprivation (a.k.a. Fasting)
Yes, the most popular diet trend of the year plays a key role in signaling autophagy. In fact, the strongest signal to the body to start autophagy may be fasting.As you know, muscle growth and repair is mediated by the mechanistic target of rapamycin (mTOR) pathway in the body. When you eat protein, this pathway is activated and without getting into the incredibly complex physiology of how it happens (it would take WAY too long), autophagy is essentially deactivated.
Even small amounts of amino acids (~10 grams) or glucose can inhibit autophagy, as both glucose and certain amino acids can raise insulin, which is a principle negative regulators of autophagy. [13] What this means, is that even a small meal or sipping on your beloved BCAA can blunt the autophagic process.
While it may sound strange, if you want to maximize autophagy, you’re going to have to put your gains on hold for a bit, as autophagy is induced by mTOR inactivated, which is caused by nutrient deprivation, specifically protein.[29]
Fasting also causes a significant drop in certain growth factors, such as insulin-like growth factor 1 (IGF-1), which also serves as a signal to the body to activate autophagy.
How long do you need to fast?
Research in mice shows that at least 24-48 hours is needed to significantly upregulate autophagy. Other research notes that a 12 hour fast was able to upregulate hepatic autophagy in mice, but other tissues were not studied.
Hypoxia
Your body doesn’t like being without oxygen, just try holding your breath underwater and see just how long it takes before you start freaking out. This lack of fresh oxygen getting delivered to your tissues serves as a powerful signal to start autophagy.Now, we’re not suggesting you experiment with self-suffocation, but depriving your body of oxygen is a powerful stressor that can trigger the cell recycling process.
Exercise
Now, this is more like it!We can’t think of a more enjoyable form of stressing the body than intense exercise. High-intensity training such as heavy weight lifting or interval training (HIIT) subject the body to incredible stress, and in response to this stress, autophagy kicks in to repair and fortify your cells so that they are stronger and more durable the next time they encounter this type of stress.
One particular study engineered the test subjects (mice) to have glowing green autophagosomes (yes this really happens) to test the effects of intense exercise on autophagy. Researchers noted that the rate of self-eating was significantly increased after 30 minutes of running, and it continued to increase up to 80 minutes. [16]
Now, this study was done in mice, and we don’t know how long or intense the training must be in humans, yet, but clearly, there is a link between exercise and autophagy. Some human research has noted that short 20-minute sub-maximal aerobic exercise (81% VO2 max) did not exert significant effects on autophagy in skeletal muscle, but ultra-endurance exercise did. [22][23][24] So, until more research is done, the link between exercise intensity and duration and autophagy is still unclear.
Go Low Carb or Keto
Animal studies using rats placed on a ketogenic diet demonstrate that autophagy is activated, and has led to reduced brain injury during and after seizures. [17]How does keto relate to autophagy?
Remember, insulin and activation of mTOR are two of the biggest autophagy inactivators, so by going keto or extremely low carb with your diet, you keep insulin levels very low and promote autophagy.
This might also be a way to increase autophagy without having to full on fast.
Restrict Protein Intake
One other means to upregulate autophagy while avoiding fasting might be able to severely reduce your protein intake, but still maintain a reasonable intake of calories.While this hasn’t been extensively studied in humans, restricting your protein intake substantially (i.e. only consuming 25-30 grams PER day) for a week may activate autophagy by causing a drop in insulin-like growth factor-1 (IGF-1) which reduces the transfer of glucose into the cell, resulting in autophagy activation.
Sleep Well
Autophagy can occur while you sleep, and as you may know, the circadian rhythm governs your sleep cycle. It also affects the autophagy rhythm, which is yet another reason why getting proper sleep is a must. [28]Drink Coffee And Green Tea (No Cream or Sugar!)
Consumption of coffee and epigallocatechin-3-Gallate (EGCG, the primary polyphenol in green) tea has been shown to increase autophagy throughout the body. [26][27] Just remember to drink it without cream or sugar, since those two things can break your fast and stimulate insulin release, both of which inactivate autophagy.Heat Exposure
The final “readily” do-able means to activate autophagy (there are several others that aren’t as easy to do) is by exposure to heat. Heat stress denatures proteins and affects translation. It also serves as a strong stimulus for the body’s “heat shock response.” Part of this response is autophagy. [20][21]Benefits of Autophagy
There are a wide range of benefits associated with autophagy including:- Slow down the aging process
- Reduce progression of neurodegenerative diseases, or prevent them outright
- Lower inflammation
- Better skin complexion
- Improve energy production
- Repair mitochondria, which is damaged by oxidative stress
- Protect the brain and central nervous system
- Improve brain structure and neuroplasticity
- Enhanced cognitive function
- Supports immune system function
- Supports cardiovascular health and function
- Protects against toxic proteins that contribute to amyloid diseases
- Protect DNA
- Potentially combat cancer
Takeaway
It’s pretty clear that in order to make ourselves stronger and more resilient, stress and suffering are a must. It’s only through breaking ourselves down that we can adapt and overcome, hence why resistance training is needed for muscle growth.Autophagy is the ultimate stress-response mechanism in the body, one that can be activated a number of ways, but all involve stress in some form or another. While none of us enjoy stressing our bodies unnecessarily, it’s pretty clear that to live longer and be stronger, stress is required.
Starvation and exercise are the two strongest stressors in the body, use one or use both, just make sure you provide adequate stress to reap the numerous benefits of autophagy and eat yourself to a stronger you!



