01dragonslayer
Iron Killer
Mad Referrer
Jacked Immortal
EG Auction Sniper
VIP Member
Shout Master
Mutated
Fully Loaded
- EG Cash
- 1,113,693
What are mitochondria?
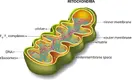 Mitochondria are membrane-bound cell organelles (mitochondrion, singular) that generate most of the chemical energy needed to power the cell's biochemical reactions. Chemical energy produced by the mitochondria is stored in a small molecule called adenosine triphosphate (ATP).
Mitochondria are membrane-bound cell organelles (mitochondrion, singular) that generate most of the chemical energy needed to power the cell's biochemical reactions. Chemical energy produced by the mitochondria is stored in a small molecule called adenosine triphosphate (ATP).Mitochondria are small, often between 0.75 and 3 micrometers and are not visible under the microscope unless they are stained.
Unlike other organelles (miniature organs within the cell), they have two membranes, an outer one and an inner one. Each membrane has different functions. Mitochondria are split into different compartments or regions, each of which carries out distinct roles.
Different cell types have different numbers of mitochondria. For instance, mature red blood cells have none at all, whereas liver cells can have more than 2,000. Cells with a high demand for energy tend to have greater numbers of mitochondria. Around 40 percent of the cytoplasm in heart muscle cells is taken up by mitochondria.
Although mitochondria are often drawn as oval-shaped organelles, they are constantly dividing (fission) and bonding together (fusion). So, in reality, these organelles are linked together in ever-changing networks. Also, in sperm cells, the mitochondria are spiraled in the midpiece and provide energy for tail motion.
What happens to our mitochondria as we age? How do they become dysfunctional?
Age-related changes in mitochondria are associated with decline in mitochondrial function. With advanced age, mitochondrial DNA volume, integrity and functionality decrease due to accumulation of mutations and oxidative damage induced by reactive oxygen species (ROS).In aged subjects, mitochondria are characterized by impaired function such as lowered oxidative capacity, reduced oxidative phosphorylation, decreased ATP production, significant increase in ROS generation, and diminished antioxidant defense. Mitochondrial biogenesis declines with age due to alterations in mitochondrial dynamics and inhibition of mitophagy, an autophagy process that removes dysfunctional mitochondria.
Age-dependent abnormalities in mitochondrial quality control further weaken and impair mitochondrial function. In aged tissues, enhanced mitochondria-mediated apoptosis contributes to an increase in the percentage of apoptotic cells. However, implementation of peptides and strategies such as caloric restriction and regular physical training may delay mitochondrial aging and attenuate the age-related phenotype in humans.
What You Need to Know
Mitochondrial disease can greatly impact one's health by negatively affecting almost any part of the body. Health concerns with mitochondrial disease include fatigue, diabetes mellitus, impairment of hearing and vision, weakness, metabolic strokes, seizures, cardiomyopathy, arrhythmias, developmental or cognitive disabilities, liver, gastrointestinal disorders, liver and kidney disease, and more. Many of these conditions can lead to secondary mitochondrial dysfunction and affect other diseases, including Alzheimer's disease, muscular dystrophy, Lou Gehrig's disease, diabetes and cancer.SS-31
What is SS-31?SS-31 is a class of cell-permeable small peptides, which selectively resides on the inner mitochondrial membrane and possesses intrinsic mitochondrial protective capacities. Specifically, SS-31 (also known as MTP-131) is a water-soluble mitochondria-targeting peptide that attenuates mitochondrial reactive oxygen species production and cytochrome c release. This peptide has been shown to reverse decline in diabetes, attenuate transverse aortic constriction-induced pulmonary arterial hypertension and heart failure, and rapidly rejuvenates oxidative phosphorylation in aged mice.
SS-31 (also known as Bendavia, Elamipretide, and MTP-131) is a small peptide (D-Arg Dimethyl-Tyr-Lys-Phe-NH2) that accumulates in mitochondria and scavenges reactive oxygen species. SS31 binds to cardiolipin, a lipid exclusively expressed on the inner mitochondrial membrane that plays an important structural role in organizing the components of the electron transport chain into “super complexes'' for more efficient oxidative phosphorylation with minimal generation of reactive oxygen species (Birk et al., 2013; Szeto, 2014). By binding to cardiolipin, SS-31 modulates the hydrophobic interaction between cytochrome c and cardiolipin and promotes the electron carrying function of cytochrome c (Szeto, 2014). SS-31 also inhibits the opening of the mitochondrial permeability transition pore that forms under mitochondrial stress (e.g., traumatic brain injury, stroke, neurodegenerative diseases) (Wu et al., 2016). Opening of the mitochondrial permeability transition pore can lead to mitochondrial swelling and apoptosis. SS-31 was discovered by Dr. Hazel Szeto at Weill Cornell Medical College.
Why would I want to explore SS-31?
SS-31 is a novel mitochondrial-target therapy peptide that crosses the blood brain barrier (BBB) and targets the inner mitochondrial membrane (IMM). Mitochondria play a vital role in cellular metabolism. Because of their widespread effects on cellular functions, mitochondrial dysfunction is implicated in a wide range of diseases. Mitochondrial dysfunction results in less ATP production and insufficient energy to maintain cell function, which is followed by cell injury and even cell death.SS-31 Research Example #1:
Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in miceBackground
It is widely accepted that mitochondria have a direct impact on neuronal function and survival. Oxidative stress caused by mitochondrial abnormalities play an important role in the pathophysiology of lipopolysaccharide (LPS)-induced memory impairment. Elamipretide (SS-31) is a novel mitochondrion-targeted antioxidant. However, the impact of elamipretide on the cognitive sequelae of inflammatory and oxidative stress is unknown.
Methods
We utilized MWM and contextual fear conditioning test to assess hippocampus-related learning and memory performance. Molecular biology techniques and ELISA were used to examine mitochondrial function, oxidative stress, and the inflammatory response. TUNEL and Golgi-staining was used to detect neural cell apoptosis and the density of dendritic spines in the mouse hippocampus.
Results
Mice treated with LPS exhibited mitochondrial dysfunction, oxidative stress, an inflammatory response, neural cell apoptosis, and loss of dendritic spines in the hippocampus, leading to impaired hippocampus-related learning and memory performance in the MWM and contextual fear conditioning test. Treatment with elamipretide significantly ameliorated LPS-induced learning and memory impairment during behavioral tests. Notably, elamipretide not only provided protective effects against mitochondrial dysfunction and oxidative stress but also facilitated the regulation of brain-derived neurotrophic factor (BDNF) signaling, including the reversal of important synaptic-signaling proteins and increased synaptic structural complexity.
Conclusion
These findings indicate that LPS-induced memory impairment can be attenuated by the mitochondrion-targeted antioxidant elamipretide. Consequently, elamipretide may have a therapeutic potential in preventing damage from the oxidative stress and neuroinflammation that contribute to perioperative neurocognitive disorders (PND), which makes mitochondria a potential target for treatment strategies for PND.
SS-31 Research Example #2:
Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged miceMoment-to-moment adjustment of cerebral blood flow (CBF) via neurovascular coupling has an essential role in maintenance of healthy cognitive function. In advanced age, increased oxidative stress and cerebro-microvascular endothelial dysfunction impair neurovascular coupling, likely contributing to age-related decline of higher cortical functions. There is increasing evidence showing that mitochondrial oxidative stress plays a critical role in a range of age-related cellular impairments, but its role in neurovascular uncoupling remains unexplored. This study was designed to test the hypothesis that attenuation of mitochondrial oxidative stress may exert beneficial effects on neurovascular coupling responses in aging. To test this hypothesis, 24-month-old C57BL/6 mice were treated with a cell-permeable, mitochondria-targeted antioxidant peptide (SS-31; 10 mg kg−1 day−1, i.p.) or vehicle for 2 weeks. Neurovascular coupling was assessed by measuring CBF responses (laser speckle contrast imaging) evoked by contralateral whisker stimulation. We found that neurovascular coupling responses were significantly impaired in aged mice. Treatment with SS–31 significantly improved neurovascular coupling responses by increasing NO‐mediated cerebro-microvascular dilation, which was associated with significantly improved spatial working memory, motor skill learning, and gait coordination. These findings are paralleled by the protective effects of SS–31 on mitochondrial production of reactive oxygen species and mitochondrial respiration in cultured cerebro-microvascular endothelial cells derived from aged animals. Thus, mitochondrial oxidative stress contributes to age-related cerebro-microvascular dysfunction, exacerbating cognitive decline. We propose that mitochondria-targeted antioxidants may be considered for pharmacological microvascular protection for the prevention/treatment of age-related vascular cognitive impairment (VCI).
MOTS-c
What is it?MOTS-c is a mitochondrial-derived peptide that is primarily used for fat loss but has also shown efficacy for muscle building, improved physical performance, and as an anti-aging peptide by reversing cellular senescence*. Interestingly, the long-lived Japanese people (population with the most extended lifespan in the world) have demonstrated the phenotypic expression and biological link between MOTS-c and an extended lifespan.
How does MOTS-c work?
MOTS-c functions to activate the mitochondrial genome, thereby increasing mitochondrial biogenesis. This process all happens through the inhibition of the methionine-folate cycle, resulting in purine synthesis and increased PCG-1 alpha and AICAR, all of which play vital roles in energy metabolism via AMP-activated protein kinase (AMPK). By stimulating AMPK, *cellular senescence is, in part, reversed.What is Cellular Senescence?
When cells are damaged, they sense their damage, and they can pause. This process is called cellular senescence or cellular arrest. Cells are programmed to do this because they don’t want to replicate with damage. Instead, they pause until the immune system can clear them. Until cleared, senescent cells secrete signals that cause harm to the body. These signals increase inflammation, exhaust stem cells, and cause the body to age more rapidly. While senescence is natural, clearing senescent cells is vital to stop the aging process.
MOTS-c Research Example #1:
The Mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance“Mitochondria are known to be functional organelles, but their role as a signaling unit is increasingly being appreciated. The identification of a short open reading frame (sORF) in the mitochondrial DNA (mtDNA) that encodes a signaling peptide, humanin, suggests the possible existence of additional sORFs in the mtDNA. Here we report a sORF within the mitochondrial 12S rRNA encoding a 16 amino acid peptide named MOTS-c (mitochondrial open-reading-frame of the twelve S rRNA -c) that regulates insulin sensitivity and metabolic homeostasis. Its primary target organ appears to be the skeletal muscle and its cellular actions inhibit the folate cycle and its tethered de novo purine biosynthesis, leading to AMPK activation. MOTS-c treatment in mice prevented age-dependent and high-fat diet-induced insulin resistance, as well as diet-induced obesity. These results suggest that mitochondria may actively regulate metabolic homeostasis at the cellular and organismal level via peptides encoded within their genome.”



