01dragonslayer
Iron Killer
Mad Referrer
Jacked Immortal
EG Auction Sniper
VIP Member
Shout Master
Mutated
Fully Loaded
- EG Cash
- 1,113,693
Insulin-like Growth Factor-1 (IGF-1), also known as Somatomedin-C, is a polypeptide growth factor that is a member of the insulin family of peptides. IGF-1 includes 70 amino acids with a molecular weight of 7649 Daltons. Similar to insulin, which has 51 amino acids, IGF-1 has an A and B chain connected by 3 disulfide bonds in a single chain. The main source of IGF-1 for adults is the liver. The Insulin-like growth factors (IGFs) are mitogenic polypeptides that stimulate the proliferation and survival of various cell types including muscle, bone, and cartilage tissue.

In recent years, new technologies have enabled many advances in the growth hormone (GH) axis (Fig 1). The secretion of GH from the anterior pituitary is regulated by GH releasing hormone (GHRH), somatostatin (GH secretion inhibiting hormone) and other hypothalamic peptides called GH secretagogues, including ghrelin. GH induces the generation of IGF-1 in the liver and regulates the production of IGF-1 in many other tissues. IGF-1 uses autocrine and paracrine signaling mechanisms to interact with its target tissues. IGF-1 then stimulates systemic body growth, producing growth-promoting effects on almost every cell in the body, including skeletal muscle, cartilage, bone, liver, kidney, nervous, skin, hematopoietic cells and lung tissue.
There are many studies reporting the anabolic and chondrogenic effects of IGF-1 on the cartilage tissue by increasing the type 2 collagen mRNA expression and proteoglycan synthesis. IGF-1 plays a critical role in the responses of muscle and bone to physical stress and is required for myocyte hypertrophy, proliferation of satellite cells, osteoblast survival, and elaboration of bone in response to tissue damage. IGF -1 promotes fat metabolism in muscle tissue while simultaneously conserving glucose and up-regulating protein synthesis in individual myocytes, with the net result being muscle hypertrophy (enlargement due to increase in cell size).

Fig 1: Metabolic effects of IGF-1. GH, and insulin under physiological conditions on their target organs. The figure summarizes schematically some of the metabolic effects that IGF-1 (blue continuous line), GH (red discontinuous line), and insulin (green dotted line) exert on the kidney (upper left), brain (upper center), skeletal muscle (left), liver (center), adipose tissue (right), and pancreas (bottom). GH growth hormone, GHRH growth hormone releasing hormone, FFA free fatty acid, IR insulin receptor substrate, IGF-1 insulin- like growth factor 1, IGFBP-1 insulin-like growth factor binding protein 1.
The effects of IGF-1 are modulated by a family of binding proteins (IGFBPs) that carry the ligand in the circulation and extracellular fluids. In the plasma, 99% of IGFs are complexed to a family of binding proteins, which modulate the availability of free IGF-1 to the tissues. There are six binding proteins, with 80-90% of IGF-1 in humans carried by IGFBP-3. IGFPB-1 is regulated by insulin and IGF-1 and IGFBP-3 is regulated mainly by GH but also to some degree by IGF-1.
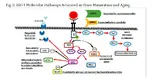
Insulin-like growth factor 1 receptor (IGF-1R) and other tyrosine kinase growth factor receptors signal through multiple pathways, including mitogen-activated protein (MAP) and P13K. A key pathway is regulated by phosphatidylinositol-3 kinase (PI3K) and its downstream partner, the mammalian target of rapamycin (mTOR). Rapamycin’s complex with FKBPP12 to inhibit the mTORC1 complex. mTORC2 remains unaffected and responds by upregulating Akt, driving signals through the inhibited mTORC1 (Figure 2).

• Conditions requiring anabolic enhancement
• Sports/athletic injury recovery
• Soft tissue repair
• Tendon/ligament/muscle repair
• Athletic or exercise performance enhancement (note this product is banned by WADA)
• Anti-aging support
• Cardiovascular conditions, including heart failure (CHF)
• Bone density improvement
• Brain/CNS issues
• Type 1 and Type 2 diabetes, Insulin resistance
• Adjunct to weight loss program; MetS
• Chronic liver disease
• Growth disorders – Acromegaly
• Helps improve connective tissue healing
• Improves brain neurogenesis, development and maturation, myelination
• Improves brain cell survival and resistance to injury
• Improves cellular repair
• Improves antioxidant system
IGF-1 levels decline as we age, and this phenomenon has been termed “somatopause” (Figure 3). Only low levels are generally detected after age 60. Improving IGF-1 levels is reported beneficial for “anti-aging” benefits. Higher IGF-1 levels are reported to improve muscle strength and mobility in older women. IGF-1 is reported to increase glutathione peroxidase, thereby improving the antioxidant defense system.

The IGF-axis helps maintain normal glucose homeostasis and keeps inflammation regulated. When IGF-1 levels are low, inflammation is high. Low levels of IGF-1 and IGFBP-3 have been associated with increased markers of inflammation, including C-reactive protein (CRP) and Interleukin-6 (IL-6). Improved IGF-1 levels are reported to decrease inflammation and decrease autoimmunity. Laboratory and human studies report inflammatory conditions, including autoimmunity, inflammatory bowel disease, multiple sclerosis, contact dermatitis, rheumatoid arthritis and lupus, are decreased when IGF-1 is administered. Immune cells, in particular macrophages, also produce IGF-1 and contribute to local tissue homeostasis. Studies have reported that lymphocyte-derived growth hormone is involved in the production of more lymphocytes and that these, in turn, can actually produce IGF-1 within the immune system.
Brain and Aging
IGF-1 is widely expressed in the central nervous system (CNS), where it promotes proliferation, survival, and differentiation of neuronal and non-neuronal cells. Microglial derived IGF-1 promotes neuronal survival. Further, IGF-1 is a potent neurotrophic factor, rescuing neurons from apoptosis and enhancing neuronal growth and myelination and prolonging survival and resistance to injury. IGF1 signaling is key in promoting organized adult hippocampal neurogenesis.
Low-dose IGF1 treatment triggered a small increase in the differentiation of neuronal progenitors into neurons. IGF1 not only promotes adult neurogenesis through increased stem cell proliferation, but also through organized cell migration Reduced IGF1 signaling is linked to cognitive dysfunction. Studies in humans found a significant correlation between better perceptual motor performance, information processing speed and fluid intelligence and higher circulating IGF1 levels. This is contradictory with some studies reporting that reduced IGF1 signaling is neuroprotective, while others claim that reduced IGF1 signaling with age contributes to brain aging. IGF1 appears to act in concert with BDNF and other neurotrophic factors to promote neurogenesis and remodeling in the brain.
Muscle Growth/Healing
Advancing age is associated with a progressive loss of skeletal muscle mass and function. IGF-1 appears to be of particular importance for the muscle regeneration process. IGF-I stimulates myoblasts proliferation and differentiation, and is implicated in the regulation of muscle growth.
In a mouse model, direct injections of human recombinant IGF-I at two, five, and seven days after injury enhanced muscle healing in lacerated, contused, and strain-injured muscles. Although IGF-I has been reported to improve muscle healing, a study reported that histology of the injected muscle revealed fibrosis within the lacerated site, despite high levels of IGF-I production.
Diabetes
Although the growth hormone (GH)-IGF-1 axis principally regulates tissue growth
and differentiation, insulin exerts its primary effects on fuel metabolism. However, these two endocrine systems interact at multiple levels and in Type 1 Diabetes, the GH-IGF-1 axis is very imbalanced, leading to increased secretion of GH, reduced plasma levels of IGF-1, and complex tissue-specific changes in IGF binding proteins (IGFBPs).
Individuals with type 1 diabetes exhibit abnormalities of the growth GH/ IGF/IGFBPs axis, including GH hypersecretion, reduced circulating levels of IGF-1 and IGFBP-3, and elevated levels of IGFBP-1. IGF-1 can promote glucose uptake in certain peripheral tissues in the magnitude of 4-7% from that of insulin. Administration of IGF-1 to patients with type 1 and 2 diabetes, insulin sensitivity is significantly improved, insulin requirements are reduced, and glycemic control of dyslipidemia is generally improved in short-term studies.
Obesity/Metabolic Syndrome (MetS)
IGF-1 signaling has been implicated in the differentiation and metabolic regulation of adipocytes. In the absence of IGF1, pre-adipocytes differentiation is prevented in vitro. This can be overcome by addition of insulin, which at sufficient concentration will activate the IGF-1 receptor. In addition, IGF-1 also can regulate adipocyte metabolism, suppressing lipolysis in a manner similar to insulin. Given that obesity is associated with adipocyte stress and death, local production of IGF-1 adipose tissue plays a critical role in response to the development of obesity.
Cardiovascular
IGF-1 is reported to have anti-inflammatory and antioxidant effects on blood
vessels, stabilizing existing plaque and reducing additional plaque accumulation. Cardiovascular disease (coronary artery disease, fatal ischemic heart disease, ischemic stroke, congestive heart failure, as well as slower recovery after a heart attack) are all associated with reduced levels of IGF-1. Low levels of IGF-I are associated with increased risk of ischemic heart disease (IHD), cardiovascular mortality, and diabetes mellitus.
Of particular note is a 2011 meta-analysis (n=12 clinical studies and 14,906 participants), reported the risk of dying from all causes was increased in subjects with low as well as high IGF-1 levels. Individuals with low IGF-1 have a 1.27 x increased risk of dying from all causes, while those with higher levels were at a 1.18 x increased risk.
Bone Density
IGF-1 and GH (growth hormone) are an essential part of skeletal growth during puberty and essential for bone health throughout life. Higher IGF-1 levels are associated with greater bone mineral density in older women. A review of clinical studies using IGF-1 administration for improving bone health reported that IGF-1 has significant anabolic activity and bone protective effects.
IGF-1 LR3
• Recombinant form of IGF-1
• 83 amino acids, molecular weight 9,111 Daltons
• IGF-1 LR3 is modified from the base IGF-1 peptide by replacing the glutamic acid at position 3 with arginine (R) – hence the R3.
• The L stands for long, as an additional 13 amino acids were added, again reducing binding, while improving metabolic stability.
• Increased half-life to 20-30 hours vs. IGF-1
• Reduced affinity for IGF binding proteins, therefore increased potency
• 3 x potency vs. IGF-1.
• IGF-1 LR3 is not recommended to use for more than 10 days consecutively.

In recent years, new technologies have enabled many advances in the growth hormone (GH) axis (Fig 1). The secretion of GH from the anterior pituitary is regulated by GH releasing hormone (GHRH), somatostatin (GH secretion inhibiting hormone) and other hypothalamic peptides called GH secretagogues, including ghrelin. GH induces the generation of IGF-1 in the liver and regulates the production of IGF-1 in many other tissues. IGF-1 uses autocrine and paracrine signaling mechanisms to interact with its target tissues. IGF-1 then stimulates systemic body growth, producing growth-promoting effects on almost every cell in the body, including skeletal muscle, cartilage, bone, liver, kidney, nervous, skin, hematopoietic cells and lung tissue.
There are many studies reporting the anabolic and chondrogenic effects of IGF-1 on the cartilage tissue by increasing the type 2 collagen mRNA expression and proteoglycan synthesis. IGF-1 plays a critical role in the responses of muscle and bone to physical stress and is required for myocyte hypertrophy, proliferation of satellite cells, osteoblast survival, and elaboration of bone in response to tissue damage. IGF -1 promotes fat metabolism in muscle tissue while simultaneously conserving glucose and up-regulating protein synthesis in individual myocytes, with the net result being muscle hypertrophy (enlargement due to increase in cell size).
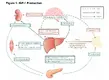
Fig 1: Metabolic effects of IGF-1. GH, and insulin under physiological conditions on their target organs. The figure summarizes schematically some of the metabolic effects that IGF-1 (blue continuous line), GH (red discontinuous line), and insulin (green dotted line) exert on the kidney (upper left), brain (upper center), skeletal muscle (left), liver (center), adipose tissue (right), and pancreas (bottom). GH growth hormone, GHRH growth hormone releasing hormone, FFA free fatty acid, IR insulin receptor substrate, IGF-1 insulin- like growth factor 1, IGFBP-1 insulin-like growth factor binding protein 1.
The effects of IGF-1 are modulated by a family of binding proteins (IGFBPs) that carry the ligand in the circulation and extracellular fluids. In the plasma, 99% of IGFs are complexed to a family of binding proteins, which modulate the availability of free IGF-1 to the tissues. There are six binding proteins, with 80-90% of IGF-1 in humans carried by IGFBP-3. IGFPB-1 is regulated by insulin and IGF-1 and IGFBP-3 is regulated mainly by GH but also to some degree by IGF-1.
Insulin-like growth factor 1 receptor (IGF-1R) and other tyrosine kinase growth factor receptors signal through multiple pathways, including mitogen-activated protein (MAP) and P13K. A key pathway is regulated by phosphatidylinositol-3 kinase (PI3K) and its downstream partner, the mammalian target of rapamycin (mTOR). Rapamycin’s complex with FKBPP12 to inhibit the mTORC1 complex. mTORC2 remains unaffected and responds by upregulating Akt, driving signals through the inhibited mTORC1 (Figure 2).
What have Research Studies Shown?
Scientific research has revealed that IGF-1 can do the following:• Conditions requiring anabolic enhancement
• Sports/athletic injury recovery
• Soft tissue repair
• Tendon/ligament/muscle repair
• Athletic or exercise performance enhancement (note this product is banned by WADA)
• Anti-aging support
• Cardiovascular conditions, including heart failure (CHF)
• Bone density improvement
• Brain/CNS issues
• Type 1 and Type 2 diabetes, Insulin resistance
• Adjunct to weight loss program; MetS
• Chronic liver disease
• Growth disorders – Acromegaly
• Helps improve connective tissue healing
• Improves brain neurogenesis, development and maturation, myelination
• Improves brain cell survival and resistance to injury
• Improves cellular repair
• Improves antioxidant system
IGF-1 in Research (Expanded)
Aging/InflammationIGF-1 levels decline as we age, and this phenomenon has been termed “somatopause” (Figure 3). Only low levels are generally detected after age 60. Improving IGF-1 levels is reported beneficial for “anti-aging” benefits. Higher IGF-1 levels are reported to improve muscle strength and mobility in older women. IGF-1 is reported to increase glutathione peroxidase, thereby improving the antioxidant defense system.

The IGF-axis helps maintain normal glucose homeostasis and keeps inflammation regulated. When IGF-1 levels are low, inflammation is high. Low levels of IGF-1 and IGFBP-3 have been associated with increased markers of inflammation, including C-reactive protein (CRP) and Interleukin-6 (IL-6). Improved IGF-1 levels are reported to decrease inflammation and decrease autoimmunity. Laboratory and human studies report inflammatory conditions, including autoimmunity, inflammatory bowel disease, multiple sclerosis, contact dermatitis, rheumatoid arthritis and lupus, are decreased when IGF-1 is administered. Immune cells, in particular macrophages, also produce IGF-1 and contribute to local tissue homeostasis. Studies have reported that lymphocyte-derived growth hormone is involved in the production of more lymphocytes and that these, in turn, can actually produce IGF-1 within the immune system.
Brain and Aging
IGF-1 is widely expressed in the central nervous system (CNS), where it promotes proliferation, survival, and differentiation of neuronal and non-neuronal cells. Microglial derived IGF-1 promotes neuronal survival. Further, IGF-1 is a potent neurotrophic factor, rescuing neurons from apoptosis and enhancing neuronal growth and myelination and prolonging survival and resistance to injury. IGF1 signaling is key in promoting organized adult hippocampal neurogenesis.
Low-dose IGF1 treatment triggered a small increase in the differentiation of neuronal progenitors into neurons. IGF1 not only promotes adult neurogenesis through increased stem cell proliferation, but also through organized cell migration Reduced IGF1 signaling is linked to cognitive dysfunction. Studies in humans found a significant correlation between better perceptual motor performance, information processing speed and fluid intelligence and higher circulating IGF1 levels. This is contradictory with some studies reporting that reduced IGF1 signaling is neuroprotective, while others claim that reduced IGF1 signaling with age contributes to brain aging. IGF1 appears to act in concert with BDNF and other neurotrophic factors to promote neurogenesis and remodeling in the brain.
Muscle Growth/Healing
Advancing age is associated with a progressive loss of skeletal muscle mass and function. IGF-1 appears to be of particular importance for the muscle regeneration process. IGF-I stimulates myoblasts proliferation and differentiation, and is implicated in the regulation of muscle growth.
In a mouse model, direct injections of human recombinant IGF-I at two, five, and seven days after injury enhanced muscle healing in lacerated, contused, and strain-injured muscles. Although IGF-I has been reported to improve muscle healing, a study reported that histology of the injected muscle revealed fibrosis within the lacerated site, despite high levels of IGF-I production.
Diabetes
Although the growth hormone (GH)-IGF-1 axis principally regulates tissue growth
and differentiation, insulin exerts its primary effects on fuel metabolism. However, these two endocrine systems interact at multiple levels and in Type 1 Diabetes, the GH-IGF-1 axis is very imbalanced, leading to increased secretion of GH, reduced plasma levels of IGF-1, and complex tissue-specific changes in IGF binding proteins (IGFBPs).
Individuals with type 1 diabetes exhibit abnormalities of the growth GH/ IGF/IGFBPs axis, including GH hypersecretion, reduced circulating levels of IGF-1 and IGFBP-3, and elevated levels of IGFBP-1. IGF-1 can promote glucose uptake in certain peripheral tissues in the magnitude of 4-7% from that of insulin. Administration of IGF-1 to patients with type 1 and 2 diabetes, insulin sensitivity is significantly improved, insulin requirements are reduced, and glycemic control of dyslipidemia is generally improved in short-term studies.
Obesity/Metabolic Syndrome (MetS)
IGF-1 signaling has been implicated in the differentiation and metabolic regulation of adipocytes. In the absence of IGF1, pre-adipocytes differentiation is prevented in vitro. This can be overcome by addition of insulin, which at sufficient concentration will activate the IGF-1 receptor. In addition, IGF-1 also can regulate adipocyte metabolism, suppressing lipolysis in a manner similar to insulin. Given that obesity is associated with adipocyte stress and death, local production of IGF-1 adipose tissue plays a critical role in response to the development of obesity.
Cardiovascular
IGF-1 is reported to have anti-inflammatory and antioxidant effects on blood
vessels, stabilizing existing plaque and reducing additional plaque accumulation. Cardiovascular disease (coronary artery disease, fatal ischemic heart disease, ischemic stroke, congestive heart failure, as well as slower recovery after a heart attack) are all associated with reduced levels of IGF-1. Low levels of IGF-I are associated with increased risk of ischemic heart disease (IHD), cardiovascular mortality, and diabetes mellitus.
Of particular note is a 2011 meta-analysis (n=12 clinical studies and 14,906 participants), reported the risk of dying from all causes was increased in subjects with low as well as high IGF-1 levels. Individuals with low IGF-1 have a 1.27 x increased risk of dying from all causes, while those with higher levels were at a 1.18 x increased risk.
Bone Density
IGF-1 and GH (growth hormone) are an essential part of skeletal growth during puberty and essential for bone health throughout life. Higher IGF-1 levels are associated with greater bone mineral density in older women. A review of clinical studies using IGF-1 administration for improving bone health reported that IGF-1 has significant anabolic activity and bone protective effects.
IGF-1 LR3
• Recombinant form of IGF-1
• 83 amino acids, molecular weight 9,111 Daltons
• IGF-1 LR3 is modified from the base IGF-1 peptide by replacing the glutamic acid at position 3 with arginine (R) – hence the R3.
• The L stands for long, as an additional 13 amino acids were added, again reducing binding, while improving metabolic stability.
• Increased half-life to 20-30 hours vs. IGF-1
• Reduced affinity for IGF binding proteins, therefore increased potency
• 3 x potency vs. IGF-1.
• IGF-1 LR3 is not recommended to use for more than 10 days consecutively.